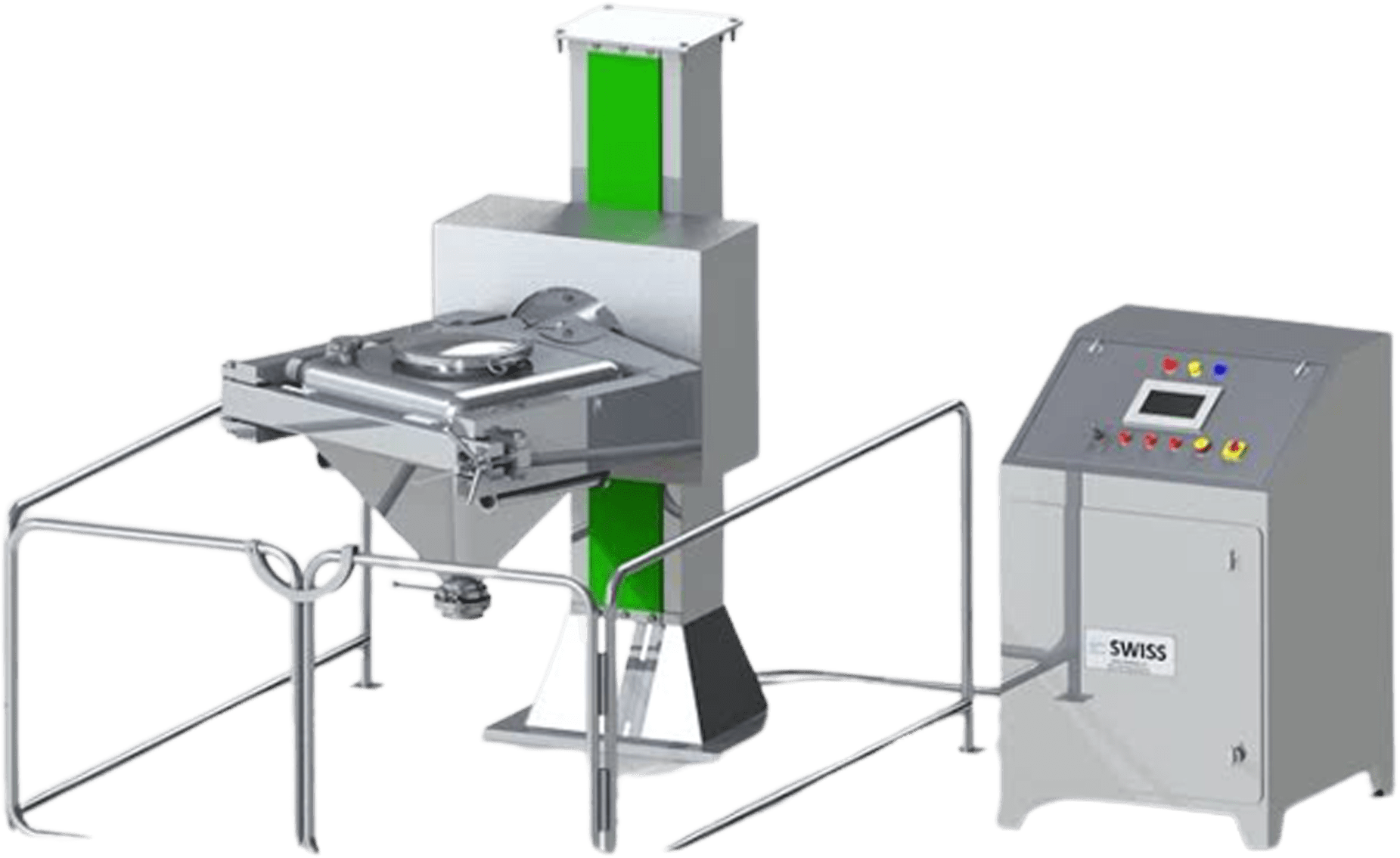
Conta-bin Blender System: Pharmaceutical Innovation
Advantages of Conta-bin Blender Systems:
Technical Specification of Conta Bin Blender
| MODEL | GROSS CAPACITY IN (LTRS) | WORKING CAPACITY IN (LTRS) | MOTOR HP | HYDRAULIC LIFTING POWER |
|---|---|---|---|---|
| MM CB-150 | 150 | 120 | 1.5 x 2 | 2 HP |
| MM CB-300 | 300 | 240 | 2 x 2 | 2 HP |
| MM CB-750 | 750 | 600 | 3×2 | 2 HP |
| MM CB-1250 | 1250 | 1000 | 7.5 x 2 | 3 HP |
| MM CB-1500 | 1500 | 1200 | 7.5 x 2 | 3 HP |
| MM CB-2000 | 2000 | 1600 | 10×2 | 3 HP |
| MM CB-3000 | 3000 | 2400 | 15 x 2 | 5 HP |
Conclusion
The Conta-bin system represents a paradigm shift in pharmaceutical blending, offering a host of advantages over traditional blending methods. With its emphasis on dust-free transfer, compliance with cGMP standards, stainless steel construction, advanced electric control panel, and versatile capacities, this innovative system has redefined the standards of pharmaceutical blending efficiency and quality.
For further exploration of Conta-bin systems and their applications in pharmaceutical manufacturing, you may find the following external resources helpful:
- Pharmaceutical Technology – Overview of Conta-bin Systems
- International Journal of Pharmaceutics – Advances in Pharmaceutical Blending Technology
- FDA Guidance – Current Good Manufacturing Practices (cGMPs) for Pharmaceutical Manufacturing
These external resources provide additional insights and information on Conta-bin systems and related topics, allowing readers to delve deeper into this transformative technology. If you require further elaboration on any aspect, please feel free to ask!
